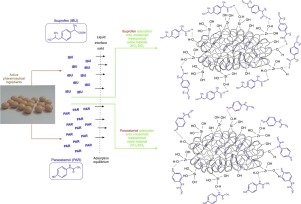
The study concerns the synthesis and application of a novel type of ZrO2-SiO2 binary oxide system. The material was prepared via a sol-gel route, using zirconium(IV) propoxide and tetraethyl orthosilicate solutions as zirconia and silica precursors, ammonia as a promoter of hydrolysis, and ethanol as a solvent. Different amounts of reactants were used to obtain samples with ZrO2:SiO2 molar ratios of 1:1, 4:1 and 1:4. The synthesized oxide materials were additionally calcined at 1000 ºC to enable the formation of a crystalline structure. Both uncalcined and calcined samples were comprehensively analyzed in terms of morphology, dispersive parameters, surface composition, crystallinity, presence of characteristic functional groups, and porous structure parameters. The proposed methodology of synthesis led to ZrO2-SiO2 oxide systems with micrometric-sized particles, interesting morphology, controlled content of zirconia and silica, and relatively high porous structure parameters. The key element of the study was an evaluation of the ability of the synthesized materials to adsorb and release selected active pharmaceutical ingredients: ibuprofen and paracetamol. It was shown that sorption capacity and release ability were strongly affected by the porosity of the materials and by the quantity of surface functional groups, which were significantly different in calcined and uncalcined samples. An appropriate mechanism of interaction was proposed.
- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact