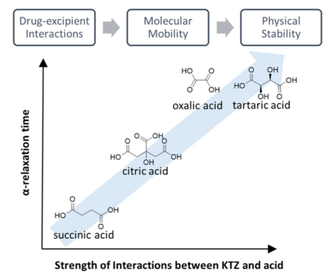
The use of excipients other than polymers for enhancing the physical stability of amorphous active pharmaceutical ingredients (APIs) has largely been unexplored. We investigated several organic acids (oxalic, tartaric, citric and succinic acid) for the purpose of stabilizing a weakly basic API, ketoconazole (KTZ), in the amorphous state. Coamorphous systems with each acid, in 1:1 KTZ-acid molar ratio, were prepared by spray drying. The interaction of KTZ with each acid was investigated by FT-IR, solid-state NMR and quantum chemical calculations. Each acid exhibited ionic and/or hydrogen bonding interactions with KTZ, and, quantum chemical calculations provided a measure of the strength of this interaction. The α-relaxation times, a measure of molecular mobility, were determined by dielectric spectroscopy, and their crystallization propensity by variable temperature X-ray powder diffractometry. Crystallization was observed only in two systems – KTZ-oxalic salt and KTZ-succinic as a cocrystal. An increase in the strength of KTZ-acid interaction translated to a decrease in molecular mobility. When the two systems prepared with structurally similar dicarboxylic acids (succinic and oxalic acid) were compared, the physical stability enhancement of KTZ-oxalic coamorphous system could be attributed to its lower mobility. However, the exceptional stability of KTZ-tartaric and KTZ-citric could not be explained by mobility alone, indicating that structural factors may also contribute to stabilization. The interaction between KTZ and acid may alter the system sufficiently so that the crystallization propensity of the KTZ-acid complex (salt or cocrystal) becomes relevant. We conclude that small molecule excipients have the potential to improve the physical stability of amorphous APIs.
- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact