Abstract
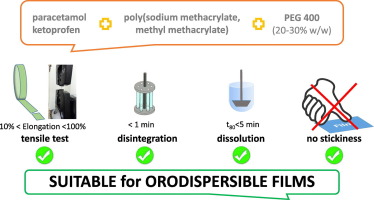
This work aims to evaluate the possible use of a poly(sodium methacrylate, methyl methacrylate) (NaPMM2) plasticized by PEG400 in design orodispersible films (ODF). Placebo ODF prepared by solvent casting were intended to study the impact of the polymer/plasticizer ratio and residual moisture on disintegration time, stickiness and mechanical properties. The drug loading capacity was assessed using ketoprofen and paracetamol. Placebo ODF containing PEG400 in the 10–30% w/w range and 10–15% of residual moisture content were easy-to-handle, packed without failures and completely dissolved within 30 s.
NaPMM2/PEG in 80/20 ratio allowed up to 70% of paracetamol loading, which appeared as the largest value described in literature. This ODF showed good mechanical properties and disintegration time. The same formulation loaded with 25% or 50% ketoprofen (pKa = 4.45) swelled without disintegrating, because of a partial protonation of NaPPM2, as verified by ATR-FTIR spectroscopy. However, the addition of 5% surfactants allowed the formulation of ODF containing 25% ketoprofen that disintegrated within one minute and guaranteed the complete drug dissolution within 5 min. All the presented data, discussed in the framework of information available on such copolymer, highlighted its versatility in the design of orodispersible dosage forms.
Conclusions
All the presented data, discussed in the framework of the information available on NaPMM2, underlined the versatility of such material that can be easily obtained by a simple salification of poly(methyl methacrylate)s that are safe materials already included in the main pharmacopeia monographs. Indeed, the surface, mechanical and dissolution properties of NaPMM2 can be modulated by adding a bivalent inorganic salt (Cilurzo et al, 2005) or a plasticizer. In the former case, it is possible to tailor the residence time of mucoadhesive dosage forms. In the latter case, their film-forming properties can be exploited to design pressure-sensitive adhesive suitable for medicated plasters (plasticizer amount higher than 40% w/w) or, as demonstrated in this work, ODF (PEG400 content lower than 30% w/w) with improved mechanical properties with respect to several polysaccharides already proposed to formulate these dosage forms. Indeed, all ODF showed satisfactory ductility, flexibility and resistance to elongation for the production, packaging and handling procedures. Last but not least, the drug loading up to 70% w/w of film weight appears to be the largest value described in literature.
Recommended for you