Abstract
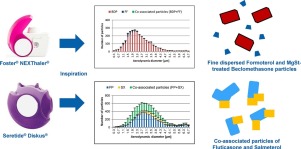
The in vitro aerosol performance of two combination dry powder inhaler (DPI) products, Foster® NEXThaler® and Seretide® Diskus® were investigated with single particle aerosol mass spectrometry (SPAMS). The in-vitro pharmaceutical performance is markedly different for both inhalers. Foster® NEXThaler® generates a higher fine particle fraction (FPF <5 μm) and a much higher relative extra fine particle fraction (eFPF <2 μm). In terms of the composition of the aerodynamic particle size distribution (APSD), it could be verified with SPAMS that overall Foster® NEXThaler® emits a significantly higher number of fine and extra fine particles with a median aerodynamic diameter (MAD) of 2.1 μm while Seretide® Diskus® had a larger MAD of 3.1 μm. Additionally, the interactions between the two active pharmaceutical ingredients (APIs) in both products are different. While Seretide® emits a significant (37%) number of co-associated API particles, only a negligible number of co-associated API particles were found in Foster® NEXThaler® (<1%). A major difference with Foster® NEXThaler® is that it contains magnesium stearate (MgSt) as a second excipient besides lactose in a so-called ‘dual excipient’ platform. The data generated using SPAMS suggested that nearly all of the beclomethasone dipropionate particles in Foster® NEXThaler® also contain MgSt and must therefore be co-associated with this additional excipient. This may help explain why beclomethasone dipropionate in Foster® NEXThaler® forms less particle co-associations with the second API, formoterol fumarate, shows a lower cohesive strength in respect to beclomethasone itself and why both APIs exhibit superior detachment from the carrier as evidenced by the increased eFPF and smaller MAD.
Conclusions
There are distinct differences in the relative in vitro pharmaceutical performance of Foster® NEXThaler® and Seretide® Diskus®. Foster® NEXThaler® generates a higher fine particle fraction for both APIs (FPF <5 μm) and furthermore also emits a significantly higher relative eFPF (extra fine particle fraction <2 μm). SPAMS was able to successfully distinguish and characterize the two different formulations in the range of 0-10 μm where a much higher number of particles with a small MAD were detected from Foster® NEXThaler® compared to Seretide® Diskus®. Furthermore, SPAMS showed that both products emit APIs in different relative particulate states and particulates with distinctly different characteristics, suggesting that the interactions between the APIs and excipients in both tested combination products are very different. The Seretide® APSD profile contained a relatively high number of co-associated API particles of FP and SX. As there is no fine lactose in the Seretide® Diskus® formulation, the co-associated particles are formed of APIs without any excipient particles involved. The Foster® NEXThaler® formulation only exhibited a negligible number of co-associated API particles of BDP and FF. Interestingly, large peaks for the presence of magnesium stearate (m/z=+24) were detected in the mass spectra of beclomethasone dipropionate in Foster® NEXThaler®. This would suggest that the BDP particles in Foster® NEXThaler® are somehow co-associated with MgSt (but not with FF). It is possible that in the manufacturing process BDP was processed (co-milled) together with MgSt to improve dispersion mechanics. Another explanation may be that the BDP or the lactose carrier are receiving a MgSt-coating that would prevent the co-association or agglomeration of the particles and moreover facilitate detachment from carrier. This would then result in high in vitro performance (eFPF) and a relatively fine APSD profile compared to a product not engineered in this way like the Seretide® formulation. It is not possible to explain this as being simply due to the presence of MgSt. However, since MgSt has been described as being a FCA in DPI formulations, it is possible that this ternary excipient is indeed capable of affecting the interparticulate forces in DPI formulations resulting in high in vitro performance.
These studies suggest that the SPAMS technique may be used to investigate the nature of API particulates and provide feedback control for the level of particle co- association (API-API or API-excipient) in early-phase development of inhaled products. A SPAMS with separate desorption and ionization lasers firing at a different wavelengths could potentially detect co-associations not only between APIs, but also between API and excipients (lactose or force control agents) [55]. This makes the SPAMS technique an attractive additional technique in the field of pharmaceutical aerosol analysis and complementary to the current standard of impactor/HPLC-analysis.
Recommended for you