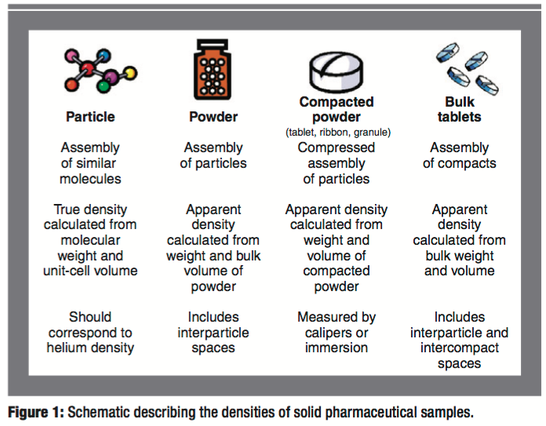
The absolute and relative densities of pharmaceutical solids play an important role in determining their performance (e.g., flow and compaction properties) in both tablet and capsule dosage forms. In this article, the authors report the densities of a wide variety of solid pharmaceutical formulations and intermediates. The variance of density with chemical structure, processing history, and dosage-form type is significant. This study shows that density can be used as an equipment-independent scaling parameter for several common drug-product manufacturing operations.
Many physical responses of powders, granules, and compacts such as powder flow and tensile strength are determined largely by their absolute and relative densities (1–8). Although measuring these properties is a simple task, a review of the literature reveals that a combined source of density data that formulation scientists can refer to does not exist. The purpose of this article is to provide such a reference source and to give insight about how these critical properties can be measured for common pharmaceutical solids and how they can be used for monitoring common drug-product manufacturing operations.
Conclusions
The authors have reported the absolute and relative densities of a wide range of pharmaceutical solids and probed some of the influences of chemical structure, processing history, and dosage-form type on these properties. Monitoring the relative density of solid pharmaceutical materials (e.g., API, excipients, blends, ribbons, granules, tablets, etc.) can be useful during the design, optimization, and scale-up of manufacturing processes for solid pharmaceutical dosage forms and may help achieve robust drug-product manufacturing processes. For example, the bulk densities of excipient and API powders will clearly indicate whether a need exists for a densifying unit operation such as dry granulation before the manufacture of tablets. Similarly, when attempting to manufacture uniform tablet dosage forms using various tablet presses, monitoring the compact relative density instead of a remote instrument parameter should provide a more robust means of ensuring consistent product performance.
Full article for download listed below