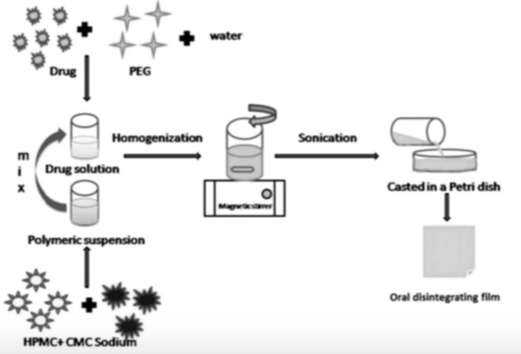
Three versatile formulations of orodispersible films (ODFs) containing donepezil hydrochloride were prepared from cellulose derivatives by solvent casting technique for the treatment of Alzheimer’s disease. The interaction between polymer and drug was investigated by spectral methods including UV, FTIR and 1H NMR and by microscopy techniques including AFM, SEM, and optical examination. Fourier transform infrared spectroscopy (FTIR) confirmed the compatibility of drug and polymer in ODF formulations. Hydrogen bonding interaction was studied by 1H NMR spectroscopy. Atomic force microscopy (AFM) of F3 films showed an average roughness of 75.34 nm and irregular particle size. According to scanning electron microscopy (SEM) data, the average particle size in F1, F2, and F3 films was 30, 37 and 50 μm, respectively. ODFs containing CMC-sodium in combination with HPMC (F3) showed faster disintegration of drug (93.01%) in comparison to F1, F2, and marketed formulation. ODF films of F3 formulation showed high surface roughness and greater pore size, which favored better and faster drug release properties.
Conclusion
We designed donepezil hydrochloride ODFs using HPMC 3 cps (F1), HPMC 5 cps (F2) and mixed HPMC – CMC form (F3). The ODF films were well charac- terized with spectral (UV, FT-IR, 1H-NMR) methods and mi- croscopic (AFM, SEM, and optical) techniques. Spectral analysis proved the presence of hydrogen bonding interac- tion between polymer and drug. This can be related with the surface roughness and pores, which were revealed by the AFM observations. According to the data of thermal analy- sis, the obtained ODF possess optimum thermal stability. Faster ODF disintegration and drug dissolution was observed for F3 formulation in comparison to F1 and F2. We conclude that this ODF formulation can be effectively used for faster drug release in the treatment of Alzheimer’s disease.
Recommended for you
- Accelerating pharmaceutical development through predictive stability approaches
- AFFINISOL™ HPMC HME for Hot Melt Extrusion
- Excipient DMF 3rd Quarter 2017