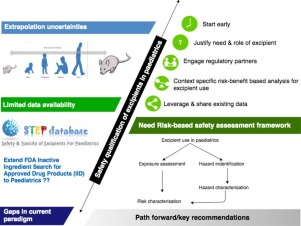
A public workshop entitled "Challenges and strategies to facilitate formulation development of pediatric drug products" focused on current status and gaps as well as recommendations for risk-based strategies to support the development of pediatric age-appropriate drug products. Representatives from industry, academia, and regulatory agencies discussed the issues within plenary, panel, and case-study breakout sessions. By enabling practical and meaningful discussion between scientists representing the diversity of involved disciplines (formulators, nonclinical scientists, clinicians, and regulators) and geographies (eg, US, EU), the Excipients Safety workshop session was successful in providing specific and key recommendations for defining paths forward. Leveraging orthogonal sources of data (eg. food industry, agro science), collaborative data sharing, and increased awareness of the existing sources such as the Safety and Toxicity of Excipients for Paediatrics (STEP) database will be important to address the gap in excipients knowledge needed for risk assessment. The importance of defining risk-based approaches to safety assessments for excipients vital to pediatric formulations was emphasized, as was the need for meaningful stakeholder (eg, patient, caregiver) Engagement.
The development of effective and safe formulation of drugs to treat pediatric patients is rife with
complexities related to the diversity of the populations (neonate to adolescent), the need for novel
technologies and approaches by Formulators to optimize efficacy of active ingredients in ways amenable to those patient populations, and the lack of mechanisms to effectively share data and experience in the scientific community. Industry drug development teams need to developing target product profiles as early as possible, understanding that the dynamics of drug development, including unanticipated (and potentially, disparate) regulatory requests for consideration of additional age groups, will require some flexibility in approach. Development of archetypes to frame constraints for various patient populations could be helpful, keeping in mind that it is the younger patient groups that set the risk posture around excipients in pediatric formations. A collaborative partnership with different disciplinary groups in regulatory agencies (eg, Chemists/Formulators, Pharmtox and Medical Reviewers, and Pediatric experts) at early pre-IND stages (US) or prior to PIP submission (EU) should be considered. However, the lack of harmonization in approach (for example, on the most appropriate age group(s) for a given therapeutic) between regulatory agencies presents further challenges. By enabling practical and meaningful discussion between scientists representing the diversity of involved disciplines (formulators, nonclinical scientists, clinicians, and regulators) and geographies (eg, US, EU), the Excipients Safety workshop session was successful in providing specific and key recommendations for defining paths forward. Leveraging orthogonal sources of data (eg. food industry, agro science), collaborative data sharing, and increased awareness of the existing sources such as the STEP database will be important to address the gap in excipients knowledge needed for risk assessment. The importance of defining riskbased approaches to safety assessments for excipients vital to pediatric formulations was emphasized, as was the need for meaningful stakeholder (eg, patient, caregiver) engagement. As development of pediatric dosage forms progresses, additional experiences with excipients will further an evolving and shared landscape for future pediatric development.
Recommended for you
- Accelerating pharmaceutical development through predictive stability approaches
- AFFINISOL™ HPMC HME for Hot Melt Extrusion
- Excipient DMF 3rd Quarter 2017