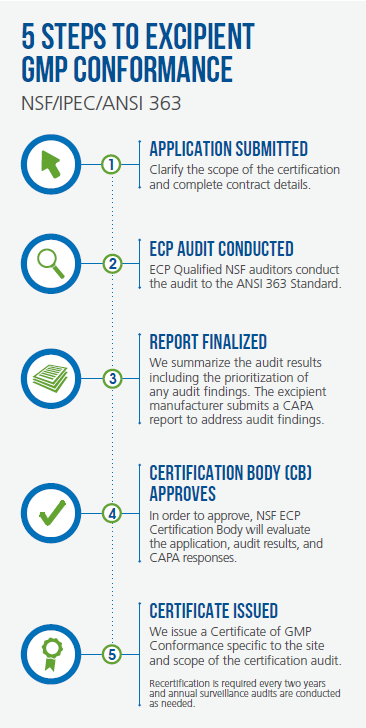
Making a case for GMP certification of an excipient manufacturer should be a straightforward
exercise since the benefits appear so clear cut and regulatory guidance in the EU underscores the
value of certification. Specifically, Chapter 3 of the EMA Guidance on formalized risk assessments to
determine the appropriate GMP for a pharmaceutical excipient states that “certification of quality systems and/or GMP by the excipient manufacturer and the standards against which these have been granted should be considered as such certification may fulfil the requirements”. Furthermore, FDA participation in the development of a consensus standard, NSF/IPEC/ANSI 363 Good Manufacturing Practices (GMP) for Pharmaceutical Excipients, reinforces agency interest in ensuring pharmaceutical excipients are manufactured to an appropriate GMP standard. This article summarizes the benefits of an excipient GMP certification program (ECP) both from the point of view of the excipient manufacturer and the excipient customer.
For more information, contact pharmamail@nsf.org or visit www.nsfpharmabiotech.org
SF International. August 2017. Advantages of Excipient GMP Certification. NSF: York, UK.
For more information, contact pharmamail@nsf.org or visit www.nsfpharmabiotech.org
Recommended for you
- Accelerating pharmaceutical development through predictive stability approaches
- AFFINISOL™ HPMC HME for Hot Melt Extrusion
- Excipient DMF 3rd Quarter 2017