Abstract
Poor water-solubility remains a typical property of drug candidates in pharmaceutical development pipelines today. Various processes have been developed to increase the solubility, dissolution rate, and bioavailability of these active ingredients belonging to biopharmaceutical classification system (BCS) II and IV classifications. Since the early 2000s, nanocrystal delivery and amorphous solid dispersions are more established techniques to overcome the limitations of poorly-water soluble drugs in FDA available products. This article provides an updated review of nanocrystal and amorphous solid dispersion techniques primarily for orally delivered medicaments. The thermodynamic and kinetic theories relative to these technologies are presented along with marketed product evaluations and a survey of commercially relevant scientific literature.
Conclusion
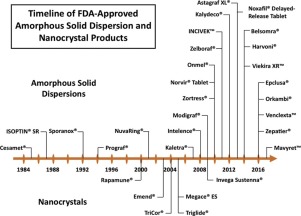
Nanocrystal delivery forms and amorphous solid dispersions are well
established techniques for addressing poor water solubility in pharmaceutical compounds, however, the methodologies are quite different. While several marketed products are made by both nanocrystal and amorphous solid dispersion technologies, it appears the number of amorphous solid dispersion products continues to grow. The trend towards increased numbers of FDA-approved amorphous solid dispersion products seems likely to continue with the introduction of new technologies to induce amorphicity, and patent literature is likely to reflect this trend in the years to come. A timeline of FDA-approved drug products and filed patents suggests solving solubility challenges for oral delivery is often successful when implementing amorphous solid dispersion technologies.
Recommended for you
- Accelerating pharmaceutical development through predictive stability approaches
- AFFINISOL™ HPMC HME for Hot Melt Extrusion
- Excipient DMF 3rd Quarter 2017