Parenteral administration of Busulfan (BU) conquers the bioavailability and biovariability related issues of oral BU by maintaining the plasma drug concentration in therapeutic range with minimal
fluctuations thereby significantly reducing the side effects. Busulfex® is the only commercially available parenteral formulation
of BU composed of organic solvents N, N-dimethylacetamide and polyethylene glycol 400. Since, BU is highly susceptible to hydrolytic degradation; Busulfex® has poor physical and chemical
stability in IV fluids. It is quintessential to develop organic solvent free formulation of BU using parenterally acceptable excipients to enhance its solubility and stability in IV fluids. The
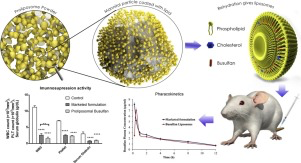
Proliposomal formulation of BU was prepared by adsorption-sonicaton method using egg phosphotidylcholine, cholesterol, tween 80 and mannitol. Vesicle size and entrapment efficiency were optimized
using 24 full factorial design and characterized by DSC, PXRD and TEM. Optimized formulation spontaneously forms 74.0±1.7 nm sized nanovesicles with 72.9±1.5 % entrapment efficiency. DSC and
PXRD studies revealed that BU was present in phospholipid bilayer in amorphized form and TEM images confirmed the multi lamellar vesicular structure. Physicochemical stability of BU was significantly
enhanced with proliposomal formulation. In-vivo studies in Sprague Dawley rats showed proliposomal formulation has comparable immunosuppression activity and 110.62 % relative
bioavailability as compared to marketed Busulfan formulation i.e. Busulfex®.