Nebulized coenzyme Q10 nanosuspensions: A pulmonary antioxidant therapy
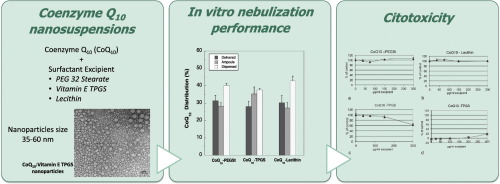
Coenzyme Q10 (CoQ10) is an antioxidant substance indicated as a dietary supplement which has been proposed as adjuvant in the treatment of cardiovascular disorders and cancer for its protective and immunostimulating activities. The aim of this work was the production by high-pressure homogenization, characterization and stability investigation of three different CoQ10 nanosuspensions designed to be administered to the lungs by nebulization.
Three surfactants, i.e. Lecithin, PEG32 Stearate and Vitamin-E TPGS, were selected to stabilize CoQ10 formulations. Preparations were identified as nanosuspensions (particle size in the range 35–60 nm): the smallest particles were obtained with Vitamin-E TPGS and denoted a core-shell structure. The CoQ10 delivered from a commercial air-jet nebulizer was in all the cases around 30% of the loaded dose. The nanosuspension containing PEG32 Stearate presented the highest respirable fraction (70.6%) and smallest MMAD (3.02 μm). Stability tests showed that the most stable formulation, after 90 days, was the one containing Vitamin-E TPGS, followed by the CoQ10-lecithin formulation. Interestingly, those formulations were demonstrated to be suitable also for nebulizers using other mechanisms of aerosol production such as ultrasound and vibrating mesh nebulizers.
Studies focused on in vitro cellular toxicity of the formulations and their single components using A549 human lung cells showed no obvious cytotoxicity for the formulations containing Lecithin and PEG 32 Stearate. Vitamin-E TPGS alone was shown to be able to damage the plasma membrane, nevertheless, cell damage was decreased when Vitamin-E TPGS was present in the formulation with CoQ10.