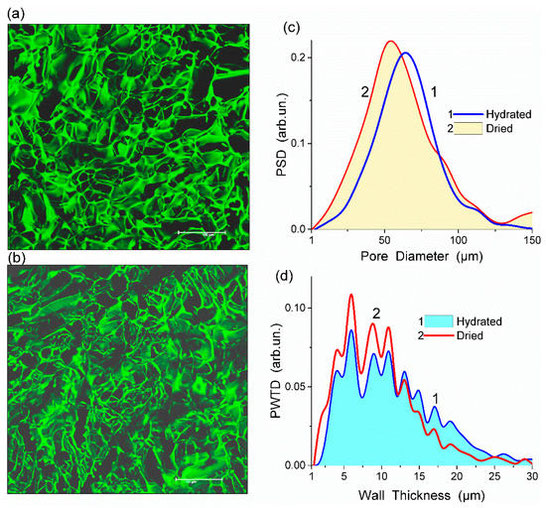
Abstract: In this review, the importance of water in hydrogel (HG) properties and structure is analyzed. A variety of methods such as 1H NMR (nuclear magnetic resonance), DSC (differential scanning calorimetry), XRD (X-ray powder diffraction), dielectric relaxation spectroscopy, thermally stimulated depolarization current, quasi-elastic neutron scattering, rheometry, diffusion, adsorption, infrared spectroscopy are used to study water in HG. The state of HG water is rather non-uniform. According to thermodynamic features of water in HG, some of it is non-freezing and strongly bound, another fraction is freezing and weakly bound, and the third fraction is non-bound, free water freezing at 0 °C. According to structural features of water in HG, it can be divided into two fractions with strongly associated and weakly associated waters. The properties of the water in HG depend also on the amounts and types of solutes, pH, salinity, structural features of HG functionalities.
Introduction: Polar, hydrophilic natural and synthetic polymers physically or chemically cross-linked into 3D-network and bonding a large amount of water (up to 100 g/g or higher), but insoluble in water are known as hydrogels (HG, water containing gels). The insolubility of HG in water is of importance for preservation of the system integrity. A simple way to solve this task is the use of chemically cross-linked polymers or macromolecules, and the degree of cross-linking more strongly affects the behavior of HG in aqueous media than the behavior of water bound in HG. The HG hydrophilicity is owed to a number of water-solubilizing groups: –OH, –COOH, –COO−, >C=O, >CHNH2, –CONH2, –SO3H, etc., in the polymer network. Water plays an important role in hydrogels by supporting their integrity, solubility and diffusion of substances, which is important for biomedical, biotechnological and environmental applications [1,2,3,4,5,6,7]. The water content and therefore the HG volume can change due to swelling/shrinking influenced by external conditions, such as temperature, pH, ionic strength, solvent nature, etc.