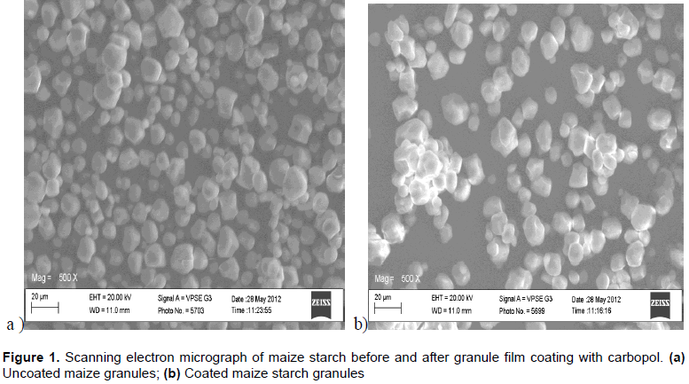
The aim of this study was to enhance the classical adjuvant functionality of maize starch (Mst) by co-processing with small quantities of carbopol 974P (CaPol). A pre-processed maize starch (MpS) was coated with different concentrations of CaPol corresponding to 0.25, 0.5, 0.75 and 1.0 %w/w and spray-dried at controlled conditions to produce the CaPol-coated motifs (MpS-CaPol). The relevant physicochemical and functional properties of the new CaPol-coated starch, as a direct compression and rapid disintegrating adjuvant were evaluated in the preparation of acetyl salicylic acid (ASA) tablets. The yield of MpS-CaPol produced with the various mix were between 87 to 95% of the weight of the start ingredients. There were no remarkable differences in the relevant physicochemical and functional properties determined for Mst and MpS thus, the results of MpS were not presented. There were no remarkable morphological differences between the particles of Mst and those of the coated motifs. The variants of the new starch motif showed properties that were sensitive to the concentrations of CaPol used in the coating. All the variants of MpS-CaPol generally showed comparative enhanced flow and moisture uptake. The swelling capacity of the new excipients containing different concentrations of CaPol can be ranked thus: 1 > 0.75 > 0.5 > 0.25 > 0% (Mst). The ASA tablets showed relative increased tensile strength and dissolution as the concentrations of CaPol increased (0 to 0.75%). The disintegration time were remarkably modified: The tablets prepared with new excipients containing CaPol of concentrations 0.25 to 0.75 %w/w all disintegrated rapidly within 1 min while that containing 1% w/w disintegrated at 45 ± 0.5 min; ASA tablets without disintegrant did not disintegrate even after 120 min. This study has shown that spray-dried Mst granules coated with small quantities of CaPol produced an excipient with new basic physicochemical properties that enhanced its adjuvant functionality when used for direct compression of rapid release ASA tablets.
DOI: 10.5897/AJPP2017.4803
Article Number: 862D0A166272
ISSN 1996-0816
Copyright © 2017
Author(s) retain the copyright of this article
http://www.academicjournals.org/AJPP