This article reviews the causes of poor bioavailability for drugs. It provides an introduction to lipid-based drug delivery systems, and how the formulation approach can be used to overcome impediments to good bioavailability of therapeutic actives, including poor water solubility, low permeability, and degradation by stomach acid or enzymes in vivo.
Introduction
Oral bioavailability, simply defined, is a measure of the quantity of drug absorbed into the systemic circulation following oral administration of drug. A variety of physicochemical and or physiological mechanisms can negatively impact the rate and extent of a drug’s oral absorption. The unfavorable physical chemical properties of a drug may include poor aqueous solubility and low dissolution rate (frequently correlated to each other),1-6 an octanol-water partition coefficient below 17 and above 3 to 5,8 which translates into poor permeability through the gastrointestinal tract wall, and a chemical structure that renders the drug molecule prone to degradation via acid catalyzed reactions within the stomach and/or via enzymes including proteases and esterases within the gastrointestinal mucosa and blood stream.9-11 The physiological mechanisms that can decrease bioavailability of orally administered drugs include pre-systemic elimination by digestive enzymes, P-glycoprotein mediated efflux transport of drugs back into the gastrointestinal tract lumen,1,18-21 and/or first-pass metabolism (hepatic elimination) whereby a drug is absorbed in the intestine and is shunted to the liver via the portal circulatory system and immediately metabolized.1,12-17
In addition to chemical modifications to the drug molecule, there are multiple formulation approaches that can be used to overcome the aforementioned obstacles and improve the bioavailability of a poorly absorbed drug. These include alteration of solution pH and/or salt formation, enteric coating, solubilization by complexation, solid dispersions, and micronization and nanoization.1,22-49 The focus of this article is the use of lipid-based formulations to overcome barriers to bioavailability.
Introduction to lipid-based dosage forms
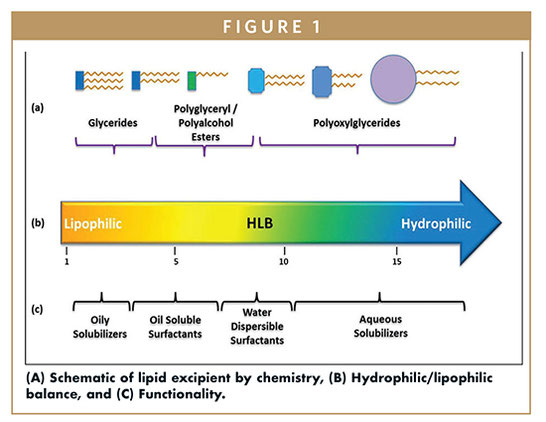
Lipid-based drug delivery systems (LBDDS) is a wide-ranging designation for formulations containing a dissolved or suspended drug in lipidic excipients. Lipids are esters of fatty acids – lipophilic hydrocarbon chains linked to a hydrophilic group like glycerol, polyglycerol, or polyalcohol (Figure 1). The melting range, solubilization capacity, and miscibility properties of the excipient are defined by the fatty acid chain length and degree of unsaturation. The amphiphilicity or dual polar and non-polar nature of lipids is characterized by the Hydrophilic Lipophilic Balance (HLB), a measure of the excipient dispersibility in aqueous media (Figure 2). Briefly, the functionality of the lipid excipients is connected to its chemistry (Figure 1).