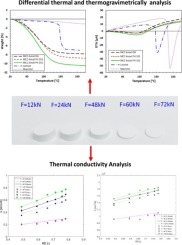
Thermal properties of powders are critical material attributes that control temperature rise during tableting and roll compaction. In this study, various analytical methods were used to measure the thermal properties of widely used pharmaceutical excipients including microcrystalline cellulose (MCC) of three different grades (Avicel PH 101; Avicel PH 102 and Avicel DG), lactose and mannitol. The effect of relative density on the measured thermal properties was investigated by compressing the powders into specimen of different relative densities. Differential thermal analysis (DTA) was employed to explore endothermic or exothermic events in the temperature range endured during typical pharmaceutical manufacturing processes, such as tabletting and roll compaction. Thermogravimetric analysis (TGA) was performed to analyse the water/solvent content, either in the form as solvates or as loosely bound molecules on the particle surface. Thermal conductivity analysis (TCA) was conducted to measure thermal conductivity and volumetric heat capacity. It is shown that, for the MCC powders, almost no changes in morphology or structural changes were observed during heating to temperatures up to 200 °C. An increase in relative density or temperature leads to a high thermal conductivity and the volumetric heat capacity. Among all MCC powders considered, Avicel DG showed the highest increase in thermal conductivity and the volumetric heat capacity, but this heat capacity was not sensitive to the measurement temperature. For lactose and mannitol, some endothermic events occurred during heating. The thermal conductivity increased with the increase in temperature and relative density. A model was also developed to describe the variation of the thermal conductivity and the volumetric heat capacity with the relative density and the temperature. It was shown that the empirical model can well predict the dependency of the thermal conductivity and the volumetric heat capacity on the relative density and the temperature.
- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact