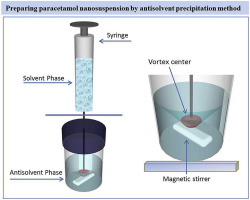
Poor aqueous solubility leading to poor oral bioavailability is a bottle neck in the development of many new drug candidates; particularly the BCS class II-IV drugs. These molecules are difficult to formulate using conventional approaches and are associated with numerous formulation related performance issues. To overcome this hurdle, formulation of nanosuspension can be one promising alternative. The purpose of this study was to develop nanosuspension using paracetamol as a model drug by antisolvent precipitation method. Nanoparticles were generated by incorporating drug solution into an antisolvent, which generated rapid nucleation leading to nanosuspension. Different stabilizers including PEG, HPMC, and Pluronic F68 were used to inhibit the particle size growth of nanosuspension. The prepared nanosuspension was evaluated in terms of particle size distribution, morphology and thermal properties. Dissolution study was also performed for nanosuspension and compared with the raw powder and commercially available paracetamol suspension (Ace® suspension). Processing conditions and type of stabilizers showed marked impact on the average particle size, morphology and stability of nanosuspension batches. Although paracetamol is sparingly soluble in water, its nanosuspension formulation exhibited faster dissolution rate when compared with commercial microsuspension and raw powder.
Recommended for you
- Reformulation of Tablets to resolve sticking & picking issues
- Captisol - modified cyclodextin for improved solubility, stability, bioavailability
- Excipient DMF List