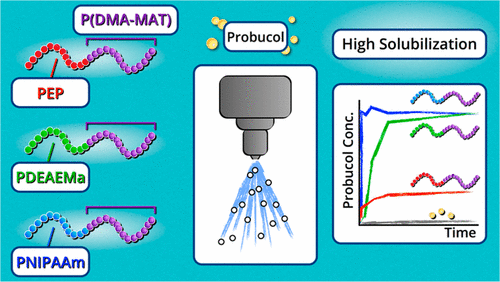
Synthetic polymers offer tunable platforms to create new oral drug
delivery vehicles (excipients) to increase solubility, supersaturation maintenance, and bioavailability of poorly aqueous soluble pharmaceutical candidates. Five well-defined diblock terpolymers were
synthesized via reversible addition–fragmentation chain transfer polymerization (RAFT) and consist of a first block of either poly(ethylene-alt-propylene) (PEP),
poly(N-isopropylacrylamide) (PNIPAm), or poly(N,N-diethylaminoethyl methacrylate) (PDEAEMA) and a second hydrophilic block consisting of a gradient copolymer
of N,N-dimethylacrylamide (DMA) and 2-methacrylamidotrehalose (MAT). This family of diblock terpolymers offers hydrophobic, hydrophilic, or H-bonding functionalities to serve as
noncovalent sites of drug binding. Drug–polymer spray dried dispersions (SDDs) were created with a model drug, probucol, and characterized by differential scanning calorimetry (DSC). These studies
revealed that probucol crystallinity decreased with increasing H-bonding sites available in the polymer. The PNIPAm-b-P(DMA-grad-MAT) systems revealed the best performance at pH 6.5,
where immediate probucol release and effective maintenance of 100% supersaturation was found, which is important for facilitating drug solubility in more neutral conditions (intestinal environment).
However, the PDEAEMA-b-P(DMA-grad-MAT) system revealed poor probucol dissolution at pH 6.5 and 5.1. Alternatively, at an acidic pH of 3.1, a rapid and high dissolution profile and
effective supersaturation maintenance of up to 90% of the drug was found, which could be useful for triggering drug release in acidic environments (stomach). The PEP-b-P(DMA-grad-MAT)
system showed poor performance (only ∼20% of drug solubility at pH 6.5), which was attributed to the low solubility of the polymers in the dissolution media. This work demonstrates the utility of
diblock terpolymers as a potential new excipient platform to optimize design parameters for triggered release and solubilizing hydrophobic drug candidates for oral delivery.
- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact