Abstract
Warfarin is intensively discussed drug with narrow therapeutic
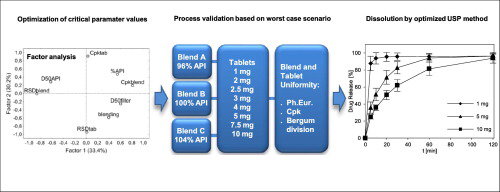
range. There have been cases of bleeding attributed to varying content or altered quality of the active substance. Factor analysis is useful for finding suitable technological parameters leading
to high content uniformity of tablets containing low amount of active substance. The composition of tabletting blend and technological procedure were set with respect to factor analysis of
previously published results. The correctness of set parameters was checked by manufacturing and evaluation of tablets containing 1–10 mg of warfarin sodium. The robustness of suggested
technology was checked by using “worst case scenario” and statistical evaluation of European Pharmacopoeia (EP) content uniformity limits with respect to Bergum division and process capability
index (Cpk). To evaluate the quality of active substance and tablets, dissolution method was developed (water; EP apparatus II; 25 rpm), allowing for statistical comparison of dissolution
profiles. Obtained results prove the suitability of factor analysis to optimize the composition with respect to batches manufactured previously and thus the use of metaanalysis under industrial
conditions is feasible.