Abstract
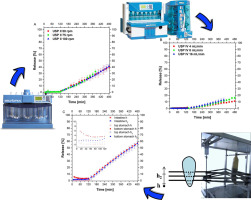
During the last decades, the study of the in vitro dissolution of pharmaceuticals has been strongly encouraged by the FDA in order to determine its relationship with the in vivo bioavailability of a drug. In this work immediate and extended release formulations containing diclofenac, a BCS class II drug, were studied using different dissolution methods. The release profiles obtained in USP Apparatus II and USP Apparatus IV were evaluated and compared to determine the effect of the fluid dynamic conditions on the release. The influence of the mixing conditions (i.e. the paddle rotation speed in USP Apparatus II or the inlet flow rate in USP Apparatus IV) on the drug release were evaluated, finding that, for the extended release formulations, they do not affect significantly the release profile. An in vitro device simulating the peristaltic contractions of the stomach during the digestion was used to simulate fluid dynamics closer to the real physiology. The tablets were found to behave in a completely different way if tested in the artificial stomach.
Both model-independent and model-dependent approaches were used to compare and fit the dissolution profiles, respectively. Fit factors were used as indicators of similarity of two dissolution profiles; model equations (such as zero-order, first-order, or Korsmeyer-Peppas equations) were used to fit the experimental data. With the identification of the best fitting model by the use of correlation factors and Akaike Information Criterion, the transport phenomena that determine the behavior of each formulation were identified.