Introduction
Multicompartment compliance aids (MCA) are widely used by patients. They support the management of medication and reduce unintentional nonadherence. MCA are filled with medicines unpacked from their original packaging. Swiss pharmacists currently provide MCA for 1–2 weeks, although little and controversial information exists on the stability of repackaged medicines.
Objective
We aimed to validate the usefulness of a simple screening method capable of detecting visual stability problems with repackaged medicines.
Methods
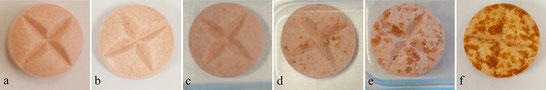
We selected eight criteria for solid formulations from The International Pharmacopoeia: (1) rough surface, (2) chipping, (3) cracking, (4) capping, (5) mottling, (6) discoloration, (7) swelling, and (8) crushing. A selection of 24 critical medicines was repackaged in three different MCA (Pharmis®, SureMed™, and self-produced blister) and stored at room temperature for 4 weeks. Pharmis® was additionally stored at accelerated conditions. Appearance was scored weekly.
Results
Six alterations (rough surface, cracking, mottling, discoloration, swelling, and crushing) were observed at accelerated conditions. No alteration was observed at room temperature, except for the chipping of tablets that had been stuck to cold seal glue.
Conclusion
The eight criteria can detect alterations of the appearance of oral solid medicines repackaged in MCA. In the absence of specific guidelines, they can serve as a simple screening method in community pharmacies for identifying medicines unsuitable for repackaging.