Abstract
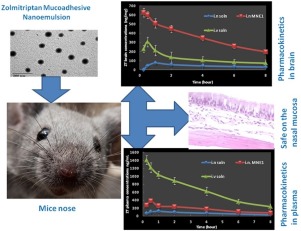
Zolmitriptan (ZT) is a well-tolerated drug in migraine treatment suffering from low bioavailability due to low amount of the drug that reaches the brain after oral and nasal delivery. Development of new nasal mucoadhesive nanoemulsion formulation for zolmitriptan may success in delivering the drug directly from the nose to the brain to achieve rapid onset of action and high drug concentration in the brain which is required for treatment of acute migraine. ZT mucoadhesive nanoemulsion were prepared and characterized for drug content, zeta potential, particle size, morphology, residence time and permeation through the nasal mucosa. The selected formula was tested in-vivo in mice for its pharmacokinetics in comparison with intravenous and nasal solution of zolmitriptan. Results showed that addition of chitosan as mucoadhesive agent in 0.3% concentration to the nanoemulsion enhanced its residence time and zetapotential with no significant effect on the globule size. All tested formulations showed higher permeability coefficients than the zolmitriptan solution through the nasal mucosa. In-vivo studies showed that the mucoadhesive nanoemulsion formulation of zolmitriptan has higher AUC0-8 and shorter Tmax in the brain than the intravenous or the nasal solution. This was related to the small globule size and higher permeability of the formulation.