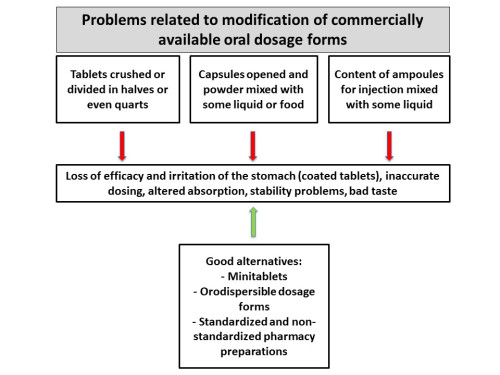
For many drugs commonly used in pediatrics, a suitable drug formulation or dosage strength is not commercially available. For that reason, children frequently receive medicines that are designed for adults, a practice called “unlicensed” or “off-label use.” The dose of commercially available products is adapted, mostly based on the child’s bodyweight, thereby neglecting differences in pharmacokinetic and pharmacodynamic parameters. Nowadays, serious attempts are being made to develop medicines, formulations, and dosage forms that are adapted to the specific needs of children. Several scientific guidelines have been released by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) concerning the development of medicines for children.
- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact