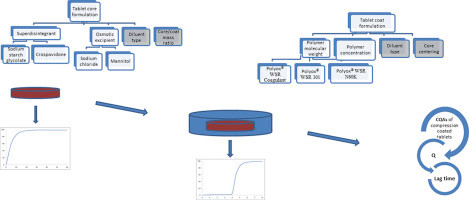
Compression coating technique has been used in formulation of chronotherapeutic drug delivery systems with pulsatile carvedilol release with polyethylene oxide as controlling release agent. FMEA, risk analysis tool, was applied within Quality by Design approach with aim to detect process and formulation parameters affecting the carvedilol release profile from compression coated tablets. It gives Risk Priority Numbers (RPNs) for each failure mode. Also, using experimental designs, statistical significance of the formulation parameters influence was estimated.